If sweet tea drinkers could read they’d be very upset by that graph.
…is what I was going to say, but man it took me a while to figure out and I’m still not 100% sure I really understand it. The specific gravity line and the sucrose vs solution line are tied to the sucrose dissolved in water curve, right? Wait, the left axis is merging two different scales? Sometimes data really isn’t beautiful.
The labels on the vertical axes match the labels on the lines. So the right vertical axis is for specific gravity (the grey line), and the left axis for the other two lines.
Ignore everything but the orange line and the left y-axis. It’s just showing the weight of sugar that fits in 100g of water, vs temperature. The blue one shows that value as a percentage, g sugar divided by total sugar and water.
Right but you’re forgetting there are already other things dissolved in the water as their not using pure, de-ionized water, and they’re adding in tea.
Tap water usually sits around 200 ppm or 0.02% minerals. The tea leaves themselves, as I make my tea, are around 10g/L. Say the leaves dissolve 10% as an overestimation. That gives you water with 0.1% tea, 0.02% other. The solubility limit for sugar is 63% (by mass).
In general, the amount of salts or other organic molecules do not affect the solubility of sugar (or any other solute). The solubility of any solute in water is a constant (for a given temperature), as long as whatever is already dissolved does not have any compounds or ions in common with the next solute.
For example, if we wanted to dissolve sodium chloride into a solution of potassium chloride, the amount of chloride already dissolved would affect the amount of NaCl we could dissolve. But if we wanted to dissolve NaCl into a solution of potassium iodide, the KI would have zero effect on the NaCl solubility.
So, since tea has zero molecules in common with the sucrose, the yes shouldn’t affect the solubility of sucrose at all. The only exception would be if solution is acidic, the sucrose can break down into glucose and fructose, of which the tea may have a small (negligible) amount.
Plus we’re not actually saturating the sweet tea. Saturated sugar water is a syrup, so you know just by the consistency that sweet tea is nowhere near saturated.
It dissolves quickly when the solution is warm. You would need to add a ridiculous amount for it to be saturated at room temp or slightly below.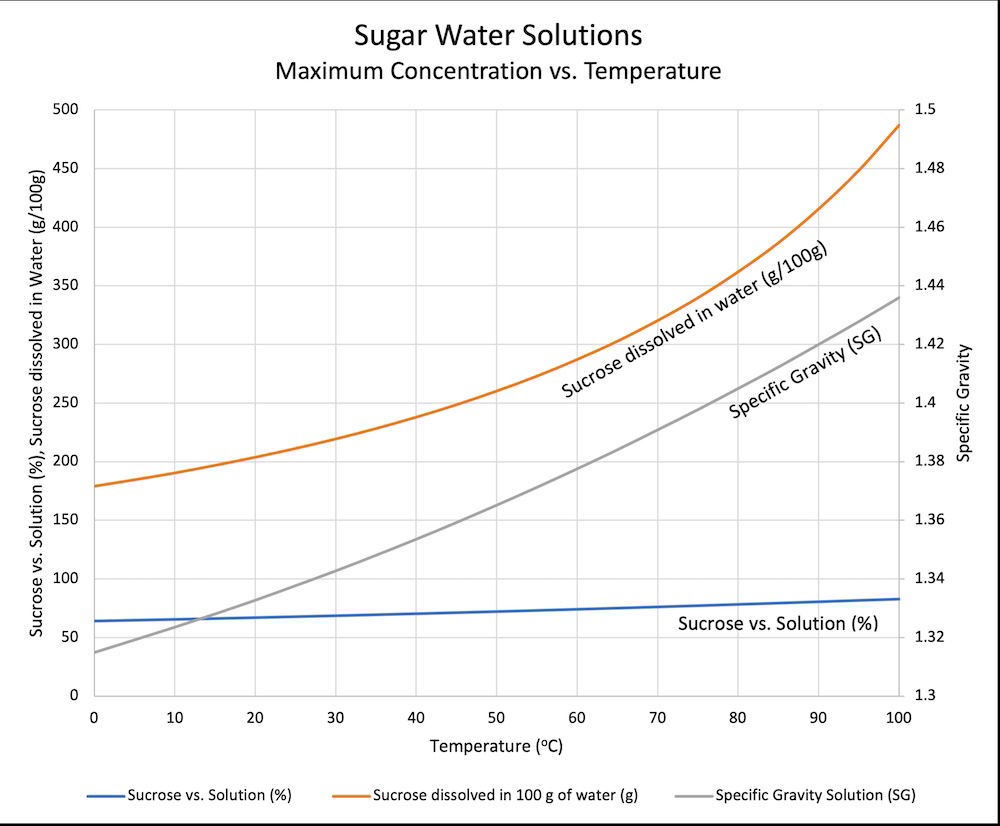
“ice cold” water can hold about 170 grams of sugar in 100 grams of water
If sweet tea drinkers could read they’d be very upset by that graph.
…is what I was going to say, but man it took me a while to figure out and I’m still not 100% sure I really understand it. The specific gravity line and the sucrose vs solution line are tied to the sucrose dissolved in water curve, right? Wait, the left axis is merging two different scales? Sometimes data really isn’t beautiful.
The labels on the vertical axes match the labels on the lines. So the right vertical axis is for specific gravity (the grey line), and the left axis for the other two lines.
Ignore everything but the orange line and the left y-axis. It’s just showing the weight of sugar that fits in 100g of water, vs temperature. The blue one shows that value as a percentage, g sugar divided by total sugar and water.
Right but you’re forgetting there are already other things dissolved in the water as their not using pure, de-ionized water, and they’re adding in tea.
I don’t think the ions and “tea molecules” really matter compared to 170g of sugar. Does a glass of water get notably heavier after adding in tea?
correct
Tap water usually sits around 200 ppm or 0.02% minerals. The tea leaves themselves, as I make my tea, are around 10g/L. Say the leaves dissolve 10% as an overestimation. That gives you water with 0.1% tea, 0.02% other. The solubility limit for sugar is 63% (by mass).
In general, the amount of salts or other organic molecules do not affect the solubility of sugar (or any other solute). The solubility of any solute in water is a constant (for a given temperature), as long as whatever is already dissolved does not have any compounds or ions in common with the next solute.
For example, if we wanted to dissolve sodium chloride into a solution of potassium chloride, the amount of chloride already dissolved would affect the amount of NaCl we could dissolve. But if we wanted to dissolve NaCl into a solution of potassium iodide, the KI would have zero effect on the NaCl solubility.
So, since tea has zero molecules in common with the sucrose, the yes shouldn’t affect the solubility of sucrose at all. The only exception would be if solution is acidic, the sucrose can break down into glucose and fructose, of which the tea may have a small (negligible) amount.
Plus we’re not actually saturating the sweet tea. Saturated sugar water is a syrup, so you know just by the consistency that sweet tea is nowhere near saturated.
Good details. Thanks Niels Bohron lol